Presentation:
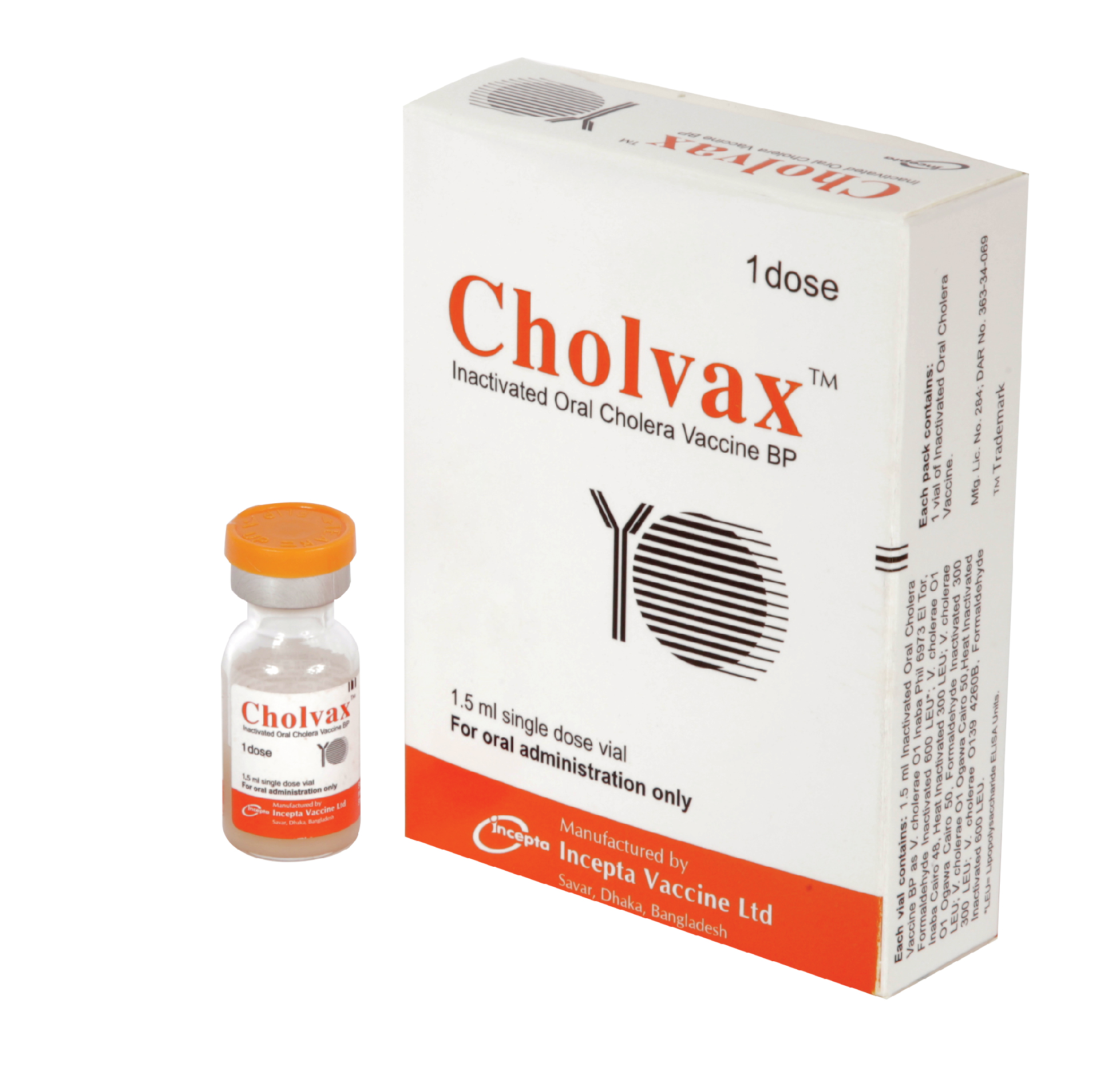
Cholvax : Each vial contains 1.5 ml Inactivated Oral Cholera Vaccine BP as-
Active ingredients:
V. cholerae O1 Inaba Phil 6973 El Tor, Formaldehyde Inactivated 600 LEU*
V. cholerae O1 Inaba Cairo 48, Heat Inactivated 300 LEU
V. cholerae O1 Ogawa Cairo 50, Formaldehyde Inactivated 300 LEU
V. cholerae O1 Ogawa Cairo 50,Heat Inactivated 300 LEU
V. cholerae O139 4260B, Formaldehyde Inactivated 600 LEU
Excipients:
Thiomersal 0.15 mg
PBS (Phosphate buffer saline) q.s to 1.5 ml
Description:
Cholvax for oral use, is a turbid, white or brownish suspension free of aggregates and extraneous particles. The vaccine is formulated with required quantity of V. cholerae O139 and O1 (serotypeInaba and Ogawa) inactivated by heat and formaldehyde.
Indications:
Cholvax is indicated for active immunization against Vibrio cholerae. The vaccine can be administered to anyone above the age of 1 year. Data for the safety and efficacy of the vaccine in infants (less than 1 year of age) is not available. The earliest onset of protection can be expected 7-10 days after the completion of the primary series of the vaccine. Efficacy against Vibrio cholerae serogroup O139 was not demonstrated.
Dosage and Administration:
The recommended dose of the vaccine (1.5 ml) is to be administered orally. The primary immunization
schedule consists of two doses given at an interval of at least two weeks. Cholera vaccine should not be administered parenterally (intramuscular, subcutaneous or intravenously). The vaccine is only recommended for oral administration.
Method of administration
The vaccine is presented as a suspension. After vigorous shaking of the vial, 1.5 ml should be poured into the mouth of the recipient. The vaccine administration may be optionally followed by water to facilitate ingestion, if needed.
The vaccine can alternatively be administered with a disposable syringe (without needle) after removing the contents from the vial and squirted into the mouth of the recipient. The vaccine should not be administered parenterally (intramuscular, subcutaneous or intravenously). The vaccine is only recommended for oral administration.
Side Effects:
The following adverse events are known to occur with cholera vaccine use. Acute
gastroenteritis, diarrhea, fever, vomiting, abdominal pain, itching, rash, nausea, weakness, cough, vertigo, dryness of mouth, oral ulcer (rare), sore throat (rare) and yellowing of urine (rare). It has been observed that the incidence of adverse events is less after the second dose as compared to the first.
Precautions:
Vaccination should be provided by a review of the medical history (especilly with regard to previous vaccination & possible occurrence of the undesirable events) and a clinical examination. As with any vaccine immunization with the cholera vaccine may not protect 100% susceptible individuals. This vaccine is also not a substitute for therapy in case of individuals suspected to be suffering from cholera or showing signs and symptoms of an acute episode of gastro intestinal disease or acute watery diarrhea. Immunocompromised persons (subsequent to a disease or immunosuppressive therapy) may not obtain the expected immune response after vaccination with the cholera vaccine. If possible, in the opinion of the medical practitioner, due consideration should be given to postpone vaccination until after the completion of the immunosuppressive treatment. As with all vaccines, appropriate medical treatment should always be available in case of a rare event of anaphylactic reactions following the administration of the vaccine. For this reason, it is recommended that the vaccinee should remain under medical supervision for at least 30 minutes after vaccination.
Contraindications:
Cholera vaccine should not be administered to subjects with either known hypersensitivity to any component of vaccine, or having shown signs of hypersensitivity after previous administration of the vaccine. Formaldehyde is used during the manufacturing process and trace amounts may be present in the final product. Caution should be taken in subjects with known hypersensitivity to formaldehyde. CVV V.N. 02 TM As with other vaccines, immunization with the cholera vaccine should be delayed in the presence of any acute illness, including acute gastrointestinal illness or acute febrile illness. A minor illness such as mild upper respiratory tract infection is not a reason to postpone immunization
Use in Pregnancy and Lactation:
No specific clinical studies have been performed to evaluate the safety and immunogenicity of cholera vaccine in pregnant women and for the fetus. However, administration of cholera vaccine to pregnant women and nursing mother may be considered after careful evaluation of the benefits and risks in case of a medical emergency or an epidemic.
Storage:
1.Keep out of the reach and sight of children.
2.Store at +2 ºC to +8 ºC. Transportation should also be at +2 ºC to +8 ºC.
3.Do not freeze. Discard vaccine if frozen.
4.Protect from light.
Commercial Pack:
Cholvax : Each box contains 1 vial of inactivated oral cholera vaccine.



.png)