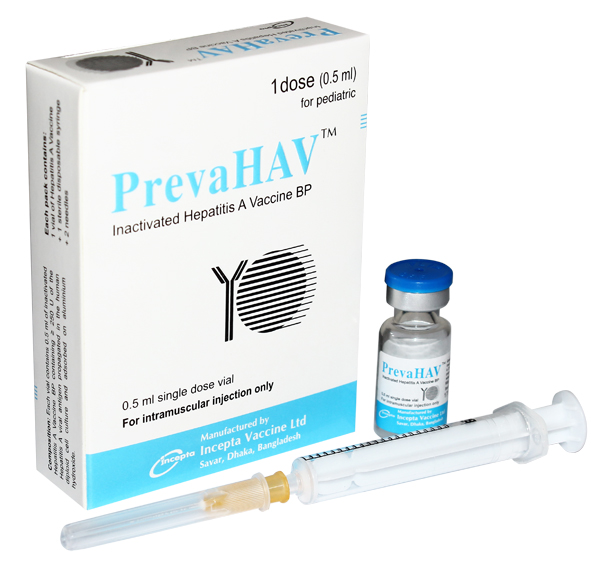
Presentation:
PrevaHAV for adult: Each vial contains 1 ml of inactivated Hepatitis A vaccine BP containing ≥ 500U of the Hepatitis A viral antigen propagated in the human diploid cell culture and adsorbed onAluminium hydroxide.
PrevaHAV for pediatric: Each vial contains 0.5 ml of inactivated Hepatitis A vaccine BPcontaining ≥ 250 U of the Hepatitis A viral antigen propagated in the human diploid cell culture and adsorbed on Aluminium hydroxide.
Description:
PrevaHAV is a purified sterile suspension of inactivated Hepatitis A vaccine derived from Hepatitis A virus cultured in human diploid cell followed by harvest, purification, inactivation by formaldehyde and Aluminium hydroxide adsorption.
Indications:
The vaccine is indicated for the active immunization of adults and children from 12 months of age against infection caused by Hepatitis A virus.
Dosage and Administration:
Hepatitis A vaccine should be given intramuscularly only. Do not inject it intravenously. The primary immunization consists of two doses, the first dose is at the selected date and the second dose will be at 6 months later.
Children 12 months to 15 years: The recommended dose is 0.5 ml
First dose: At the elected date
Second dose: 6 months after the first dose
Children 16 years and above: The recommended dose is 1 ml
First dose: At the elected date
Second dose: 6 months after the first dose
Method of administration
Hepatitis A vaccine should be injected intramuscularly in the deltoid region in adults and children and in the anterolateral part of the thigh in young children under 1 year. The vaccine should not be administered intramuscularly in the gluteal region or subcutaneously/intradermally since
administration by these routes may result in less antibody response than optimal. Hepatitis A vaccine should be inspected visually for any foreign particulate matter and/or discoloration prior to administration. Before use, the vial should be well shaken to obtain a slightly milky-white suspension. The vaccine must be used as supplied.
Side Effects:
Reactions at the site of injection are common but can be recovered within 72 hours without any treatment. Some of the mild and temporary adverse reactions are: pain and redness at the site of injection, fever after vaccination.
Precautions:
1. Shake well before use
2.The product should be used with caution for people who or whose family has the history of convulsion or epilepsy, chronic diseases and allergic symptoms
3.Do not use the product if any crack of the container, foreign particulate matter, or out of expiry date
4.This product should be used immediately after opening
5.Adrenaline and other first aid medicines should be prepared at the vaccination place in serious allergic reaction. The people after vaccination should remain under medical supervision for 30
minutes
6.The vaccine must not be frozen
Contraindications:
1. People who are allergic to any component of the vaccine
2. People who suffer from serious diseases, fever, and any immune disease
Use in Pregnancy and Lactation:
P r e g n a n c y: The vaccine should be given to a pregnant woman only if clearly needed.
L a c t a t i o n: The vaccine should be used with caution for lactating women.
Over Dosage:
Not applicable.
Storage:
• Keep out of the reach and sight of children
• Store and transport at +2 OC to +8 OC
• Protect from light
Commercial Pack:
PrevaHAV for adult: Each box contains 1 vial containing 1 ml inactivated Hepatitis A Vaccine BP
and 1 sterile disposable syringe.
PrevaHAV for pediatric: Each box contains 1 vial containing 0.5 ml inactivated Hepatitis A
Vaccine BP, 1 sterile disposable syringe and 2 needles.



.png)