Presentation:
Hepa-B for pediatrics: Each 0.5 ml contains Hepatitis B Vaccine (rDNA) BP containing ?10 µg of Hepatitis B surface antigen adsorbed on aluminum hydroxide gel equivalent to Al3+ 0.25 mg. Thiomersal 0.025 mg as a preservative. Hepa-B for adults: Each 1 ml contains Hepatitis B Vaccine (rDNA) BP containing ?20 µg of Hepatitis B surface antigen adsorbed on aluminum hydroxide gel equivalent to Al3+ 0.5 mg. Thiomersal 0.05 mg as preservative.
Description:
Hepa-B is a noninfectious recombinant DNA Hepatitis B vaccine, available in 0.5 ml and 1 ml strengths. It is a sterile suspension of the purified surface antigen of the hepatitis B virus obtained by culturing genetically engineered yeast cells of Pichia pastoris, which carry the gene that codes for the HBsAg. The HBsAg protein expressed in Pichia pastoris cells is purified by several physicochemical steps and formulated as a suspension of the antigen adsorbed on aluminium hydroxide. No substances of human origin are used in its manufacture.
Indications:
Hepa-B is indicated for active immunization against infection caused by all known subtypes of the Hepatitis B virus. As hepatitis D (caused by the delta virus) does not occur in the absence of Hepatitis B infection, it can be expected that Hepatitis D will also be prevented by Hepatitis B vaccination.
Immunization is recommended in persons of all ages, especially those who are, or will be, at increased risk of exposure to the Hepatitis B virus, for example:
• A baby whose mother is infected can be infected at birth
• Children, adolescents, and adults can become infected by:
• Contact with blood and body fluids through breaks in the skin such as bites, cuts, or sores
• Contact with objects that have blood or body fluids on them such as toothbrushes, razors or monitoring and treatment devices for diabetes
• Having unprotected sex with an infected person
• Sharing needles when injecting drugs
• Being stuck with a used needle
• Household contacts of people infected with Hepatitis B
• Residents and staff in institutions for the developmentally disabled
• Kidney dialysis patients
• People who travel to countries where Hepatitis B is common
• People with HIV infection
• Persons with hemophilia, thalassemia, sickle cell anemia, cirrhosis
• Military personnel identified as being at increased risk
• Morticians and Embalmers
• Prisoners
• Users of illicit injectable drugs
• Others: police, fire department personnel, who render first aid or medical assistance, and any others who, through their work or personal life-style, may be exposed to the Hepatitis B virus.
Dosage and Administration:
Administration
The recommended dose of vaccine (0.5 ml or 1 ml) is to administered intra-mascularly.
Dose
Neonates, infants and children upto 19 years of age: The recommended dose of Hepatitis B vaccine (rDNA) is ?10 µg of antigen protein in 0.5 ml. Adults 19 years of age and older: The recommended dose of Hepatitis B vaccine (rDNA) is ?20 µg of antigen protein in 1 ml.
Primary immunization schedule for all ages:
The usual immunization schedule consists of 3 doses of vaccine:
• First dose: at elected date
• Second dose: 1 month after first dose
• Third dose: 6 months after first dose
or
Accelerated schedule consists of 4 doses of vaccine-
• First dose: at elected date
• Second dose: 1 month after first dose
• Third dose: 2 months after first dose
• Fourth dose: 12 months after first dose
An accelerated schedule confers protection more quickly and is expected to provide better patient compliance. Neonate born to hepatitis B surface antigen-positive mother, 4 doses of 10 micrograms:
• First dose: at birth with Hepatitis B immunoglobulin injection (separate site)
• Second dose: 1 month after first dose
• Third dose: 2 months after first dose
• Fourth dose: 12 months after first dose
For travellers departing within 1 month, adult over 18 years
• First dose: at elected date
• Second dose: 7 days after first dose
• Third dose: 21 days after first dose
• Fourth dose: 12 months after first dose
Renal insufficiency (including haemodialysis patients), adult and child over 16 years 4 doses of 40 micrograms-
• First dose: at appropriate date
• Second dose: 1 month after first dose
• Third dose: 2 months after first dose
• Fourth dose: 6 months after first dose
Immunization schedule and booster doses may need to be adjusted in those with low antibody concentration.
Booster vaccinations:
For persons with normal immune status who have been vaccinated, booster doses of Hepatitis B vaccine has not been established. However, booster doses are recommended for hemodialysis patients or other immunocompromised persons.
Side Effects:
Hepatitis-B vaccine is generally well tolerated.Most of recipients of Hepatitis B vaccine experience some reaction upon vaccination.
Systemic adverse effects
• Very Common: ? 10% : Irritability , headache ,fatigue
• Common: ? 1% and < 10% : Appetite loss, drowsiness, dizziness, gastrointestinal symptoms (such as nausea, vomiting, diarrhea, abdominal pain), malaise,fever (?37.5 ºC).
• Uncommon: ? 0.1% and < 1% : Influenza-like illness, myalgia
• Rare: ? 0.01% and < 0.1% : Lymphadenopathy , arthralgia.
Local adverse effects
• Very Common: ? 10% : Pain and redness at the injection site
• Common: ? 1% and < 10% : Swelling at the injection site, injection site reaction (such as induration).
• Rare: ? 0.01% and < 0.1% : Paraesthesia, rash, pruritus, urticaria.
Precautions:
• As with all injectable vaccines, appropriate medical treatment and supervision should always be readily available in case of a rare anaphylactic reaction following the administration of the vaccine. Hepa-B vaccine should not be administered in the gluteal region or intradermally since these routes of administration may result in a lower immune response. Intradermal administration may also result in severe local reactions.The vaccine must never be administered intravenously.
• A new sterile syringe and a new sterile needle should always be used to prevent the transmission from one subject to another of infectious agents, such as the hepatitis B virus, non-A, non-B hepatitis virus or the human immunodeficiency virus (HIV).
• Patients with chronic liver disease or hepatitis C carriers should not be precluded from vaccination against hepatitis B. The vaccine could be advised since hepatitis B virus (HBV) infection can be severe in these patients. The HBV vaccination should be considered on a case by case basis by the physician.
• Patients who develop symptoms suggestive of hypersensitivity after an injection should not receive further injections of Hepa-B
• The immune response to hepatitis B vaccine is related to a number of factors, including older age, male gender, obesity, smoking habits and route of administration. In subjects who may respond less well to the administration of the hepatitis B vaccine (e.g. more than 40 years of age, individuals with type 2 diabetes, etc.), additional doses may be considered.
• Patients with HIV infection should not be precluded from vaccination against hepatitis B. The vaccine could be advised since hepatitis B virus (HBV) infection can be severe in these patients. The HBV vaccination should be considered on a case by case basis by the physician
• In HIV infected patients and persons with an impaired immune system, adequate antiHBs antibody titers may not be obtained after the primary immunization course and such patients may therefore require administration of additional doses of vaccine .
• Syncope (fainting) can occur following, or even before, any vaccination as a psychogenic response to the needle injection. It is important that procedures are in place to avoid injury from faints.
• In hemodialysis patients, adequate anti-HBs antibody titers may not be obtained after the primary immunization course and such patients may therefore require administration of additional doses of vaccine.
• The potential risk of apnoea and the need for respiratory monitoring for 48-72 hours should be considered when administering the primary immunization series to very premature infants (born ? 28 weeks of gestation) and particularly for those with a previous history of respiratory immaturity. As the benefit of vaccination is high in this group of infants, vaccination should not be withheld or delayed.
Contraindications:
Hepa-B (hepatitis B vaccine (recombinant)) is contraindicated in patients with known hypersensitivity to any component of the vaccine or having shown signs of hypersensitivity after previous Hepa-B administration.
Hepa -B should not be administered to subjects with severe febrile infections as for any vaccine. However, the presence of a minor infection does not contraindicate vaccination. Human immunodeficiency virus (HIV) infection is not considered as a contraindication for hepatitis B vaccination
Use in Pregnancy and Lactation:
Pregnancy: The effect of Hepatitis B on fetal development or reproduction capacity has not been evaluated. However, it should only be used during pregnancy when there is a high risk of infection. Lactation: Adequate human data on use during lactation and adequate animal reproduction studies are not available. It may be administered to nursing mothers only if clearly needed.
Fertility: No fertility data available
Over Dosage:
NA
Withdrawal Period:
NA
Storage:
• Keep out of the reach and sight of children
• Store at +2 ºC to +8 ºC. Transportation should also be at +2 ºC to +8 ºC
• Protect from light
• Do not freeze
Commercial Pack:
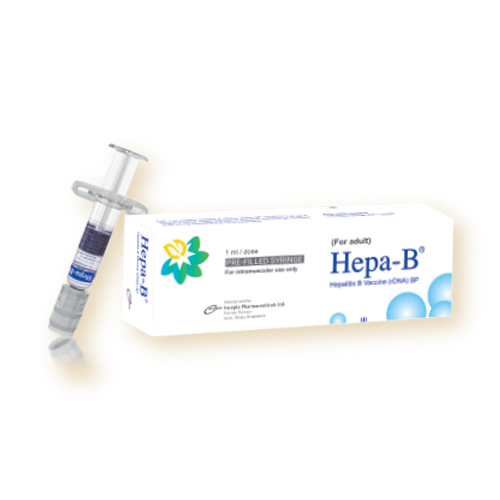
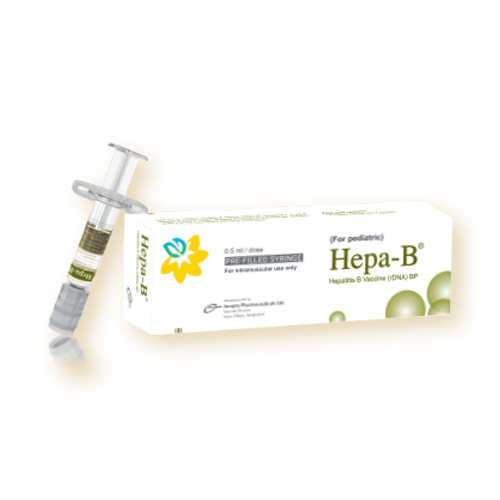
Hepa-B for pediatric: Each box contains 1 Pre-filled syringe of Hepatitis B Vaccine (rDNA) and 2 needles
Hepa-B for adult: Each box contains 1 Pre-filled syringe of Hepatitis B Vaccine (rDNA) and 1 needle.



.png)